Basic Approach
At the Maruha Nichiro Group, we operate under a quality assurance system based on the Maruha Nichiro Group Philosophy and the Maruha Nichiro Quality Assurance Policy. We prioritize the perspective of our customers, considering the “quality” they seek. Our goal is to deliver safe products and pursue food that ensures our customers' peace of mind.
To this end, we implement various measures in all processes from product development to delivery to customers, and promote quality education for employees to improve the level of knowledge and skills of each employee and raise their awareness of quality.
Management Structure
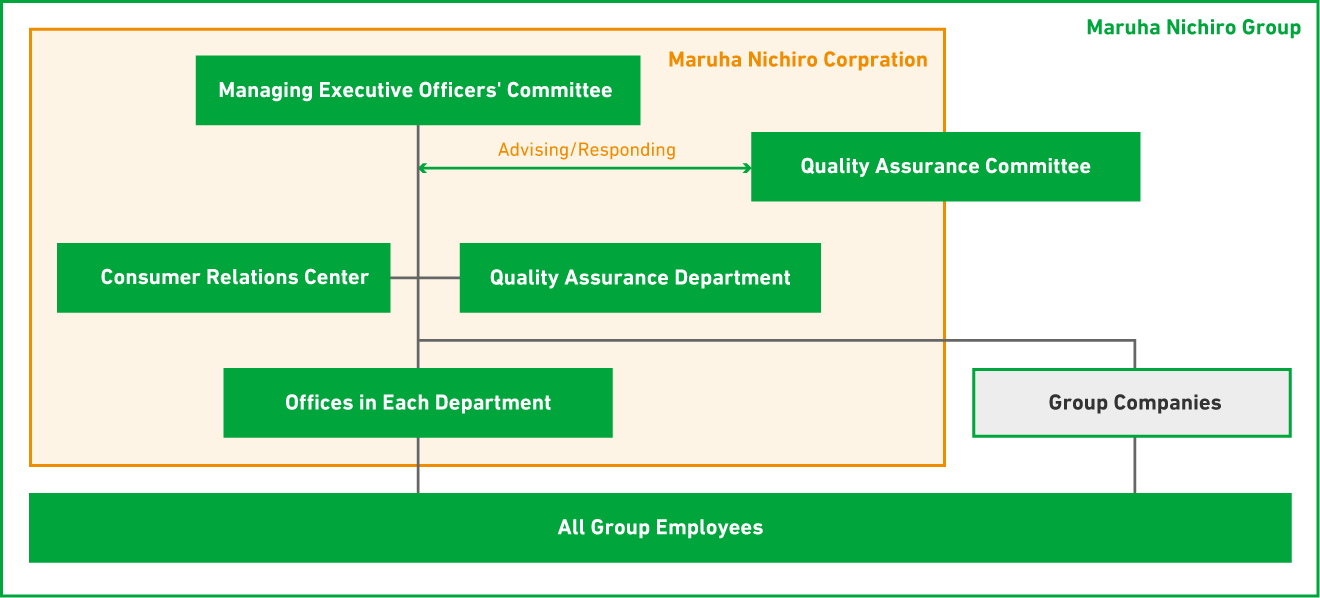
The Maruha Nichiro Group has established a system in which the Management Committee is the highest decision-making body for quality assurance, and it determines important policies and measures for implementing appropriate quality assurance activities. In addition, the Quality Committee has been established as an advisory body to the Management Committee to deliberate on matters of consultation. The Quality Assurance Department of Maruha Nichiro Co., Ltd., a core operating company, plays a key role in the Group's quality assurance, including formulating various policies, monitoring and improving the management status through the development of factory audits and product information management systems, collecting and disseminating information on quality and labeling, and providing education and awareness.
Each department in Maruha Nichiro and each group company has a "quality manager and quality officer" who communicates information on the Group's quality assurance policies and measures, as well as is responsible for planning and promoting quality assurance plans and measures.
In addition, in order to realize the Maruha Nichiro Group's purpose and mission, the Maruha Nichiro Group Quality Assurance Regulations based on ISO 9001 stipulate matters related to quality, and members of the Quality Assurance Department check the status of operations.
Quality Assurance Structure
2030 Vision (KGI) and Achievement Goals (KPIs)
Materiality: Provision of safe and secure food
KGI (the figure which wants to have 2030): Providing safe food to people around the world
Responsibility Department: Maruha Nichiro Sustainability Department (former Corporate Planning Department sustainability group)
| KPI | Target | Result | ||
|---|---|---|---|---|
| Year | Value | Achievements | Self-assessment | |
| Serious quality-related incidents*1 (Domestic G*2) | 2024 | Zero | Zero cases | ★★★★★ |
★★★★★ : KPI for FY2030 achieved.
★★★★☆ : Progress toward achieving KPI for FY2030 in advance.
★★★☆☆ : KPI for FY2024 achieved or progress toward achieving KPI for FY2030 as planned.
★★☆☆☆ : KPI delayed.
*Serious quality incidents are indicated in GRI Standard 416: Customer Health and Safety “Disclosure 416-2 Violations Concerning the Health and Safety Impact of Products and Services” and 417: Marketing and Labeling “Disclosure 417-2 Violations of Product and Service Information and Labeling” Subject to product recalls announced in newspapers or on the company's website for reasons of violation of relevant regulations and voluntary norms.
Occurrence of Serious Quality Accidents in the Past Four Years
| FY2021 | FY2022 | FY2023 | FY2024 | ||
|---|---|---|---|---|---|
| Total accidents | 4 | 4 | 0 | 0 | |
| Products quantity* | 74,229 | 18,146 | |||
*Total of individual packaging units based on internal reportings
Main Initiatives in FY2024
Continuing from the previous fiscal year, the Maruha Nichiro Group achieved zero serious quality accidents *1 in FY2024.
With the aim of providing safe and secure food and achieving zero serious quality accidents, the Group has strengthened audits and guidance at its outsourced manufacturing factories, and has obtained and maintained certification for the food safety management system at its Group production sites*2. In the external audit of the food safety management system that was reviewed by the Group's production sites in FY2024, there were no serious findings, and all minor issues were made within the deadline.
On the other hand, although we have not been able to collect company notices, the issue is to reduce complaints and troubles that can lead to serious accidents if one wrong step is made. Since January 2022, we have been working to reduce the quality risks that have become apparent by implementing zero-quality accident activities with the aim of preventing the occurrence of serious quality accidents.
By 2027, our goal is to "shift from preventing recurrence to preventing it before it happens." We have established a system to prevent abnormalities and problems from the upstream process of manufacturing, and we are working to shift to a system that "takes action before abnormalities occur." With the awareness of each and every employee as the driving force, we will strengthen our activities to instill quality-first awareness throughout the company.
- *1
- Serious quality incidents are indicated in GRI Standard 416: Customer Health and Safety "Disclosure 416-2 Violations Concerning the Health and Safety Impact of Products and Services" and 417: Marketing and Labeling "Disclosure 417-2 Violations of Product and Service Information and Labeling"Subject to product recalls announced in newspapers or on the company's website for reasons of violation of relevant regulations and voluntary norms
- *2
- Maruha Nichiro Group GFSI certification sites: 33 (as of July 2025)
Standardizing Management Method for Product Specification Information
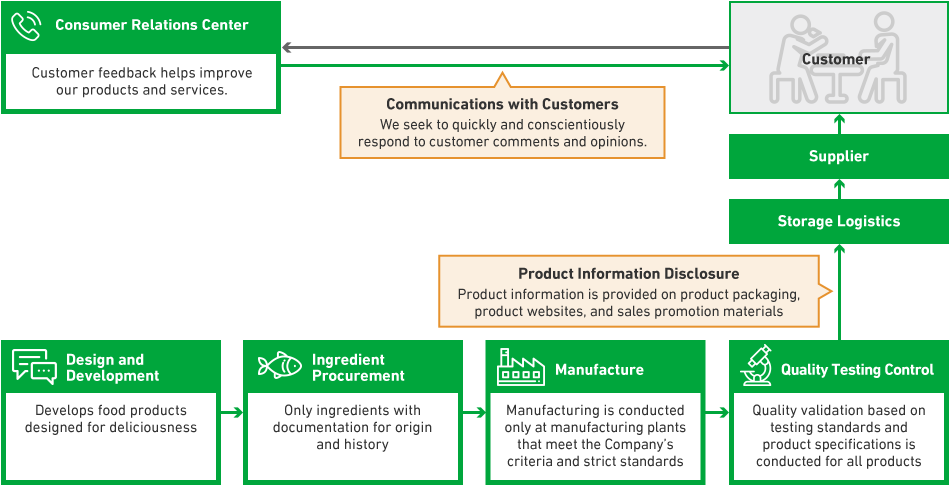
As Maruha Nichiro carries a wide range of product categories from foods that are close to raw materials, such as marine products, meat products, and agricultural products, to processed food that is consumed in a variety of settings, we have long faced the issue of having different methods for managing product specification information for different product categories. At the same time, the environment surrounding the food industry changes frequently, so there is a need to manage the latest product specification information and provide product information to customers promptly. In response to this, we have progressively introduced a new system that allows us to carry out the centralized management of everything from raw material information to product specification information and product information that we provide to customers. By utilizing this system, we will seek to provide accurate product information to customers and improve the efficiency of internal operations.
Promote Quality Education and Training
Overview of Quality Education and Training
In order to improve the quality level of the value chain provided by the Maruha Nichiro Group, it is essential to raise the awareness and knowledge of quality among all employees. In fiscal 2024, we will continue to strive to ensure the quality competence of our employees, and we will conduct a total of 40 quality-related training sessions by level and task, with a total of 16,151 people taking participating.
In the future, we will further enhance the content and improve the quality of existing content to improve the level of education and training, as well as the awareness and knowledge of employees.
| Type of training | Total sessions | Participants | Participating companies |
|---|---|---|---|
| Food label training (learning about universal guidelines, frozen food, shelf-stable food, seafood products, livestock products and practicing food label) | 12 times | 917 people | 35 |
| Quality management workshops (factory hygiene management) | 5 times | 490 people | 20 |
| Food defense workshops | 7 times | 649 people | 15 |
| FSSC/ISO22000 standards study session | 1 time | 353 people | 12 |
| FSSC 22000 internal auditor training (introductory training, skill advancement training) | 4 times | 98 people | 10 |
| Food bacteria training | 2 times | 678 people | 19 |
| Skills training for food bacteria inspectors | 1 time | 28 people | 10 |
| Practical inspection skills training for food bacteria inspectors | 2 time | 8 people | 5 |
| Customer service training | 3 times | 912 people | 9 |
| Customer feedback monitoring training | 95 departments | 11,872 people | 29 |
| Customer service training | 2 times | 146 people | 26 |
Strengthening Factory Audits and Guidance
We ensure food safety and food defense by conducting audits, reviews, and guidance in the form of visits to manufacturing sites based on our proprietary standards that take into account the requirements of GFSI-recognized certification schemes (food safety management systems). In FY2024, 17 auditors conducted audits of approximately 130 contract production plants.
In addition, we are expediting the provision of food not only within Japan but also globally. In order to respond promptly to qualityrelated issues arising from this, we have stationed quality assurance personnel in China and Thailand and are working on further strengthening our quality assurance systems in cooperation with local staff while maintaining close contact with each country/ region.

The inspection focuses on the production lines of certified factories and confirms sanitary management conditions through question-and-answer sessions with employees.

